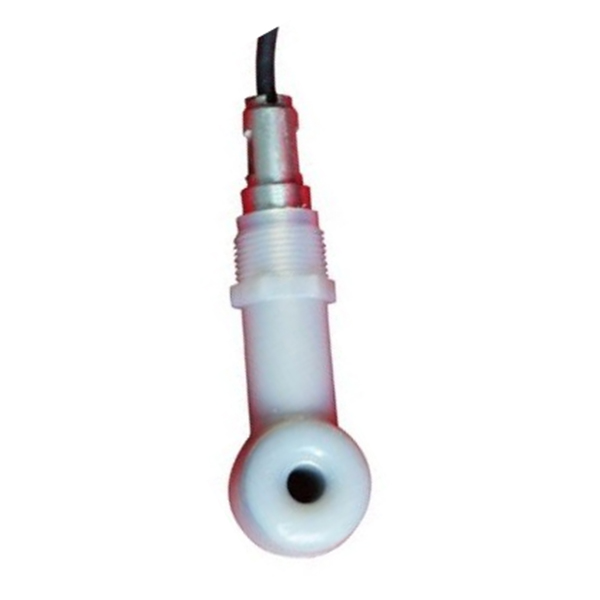
Izici
1. Ukusebenza kahle ezindaweni zamakhemikhali ezinzima kuhle kakhulu, izinto ezimelana namakhemikhali ezenziwe yi-electrode aziphazamiseki, ukugwema ukungcola, ukungcola ngisho nokuthinta izehlakalo zokumboza ungqimba olungcolile ezifana nokubi kakhulu, okulula futhi okulula ukukufaka ngakho-ke kuluhlu olubanzi kakhulu lwezicelo. Ama-electrode okuklama asetshenziswa endaweni equkethe ama-asidi amaningi (njenge-fuming sulfuric acid).
2. Ukusetshenziswa kwemitha yokuhlushwa kwe-asidi yesiNgisi, ukunemba okuphezulu, kanye nokuqina okuphezulu.
3. Ubuchwepheshe bezinzwa zokuqhuba buqeda amaphutha okuvaleka kanye nokuhlukaniswa kwe-polarization. Ukusetshenziswa kuzo zonke izindawo zama-electrode okuxhumana kungabangela ukuvaleka okunamandla aphezulu.
4. Inzwa enkulu yokuvula, ukuzinza kwesikhathi eside.
5. Faka ama-bracket ahlukahlukene futhi usebenzise isakhiwo sokufaka esivamile se-bulkhead, ukufakwa okuguquguqukayo.
1. Ukucindezela okuphezulu (ibha): 1.6MP
2. Izinto zomzimba ze-electrode: PP, ABS, PTFE ozikhethela
3. Ububanzi bokulinganisa: 0 ~ 10ms, 0 ~ 20ms, 0 ~ 200ms, 0 ~ 2000ms
4. Ukunemba (ukuqina kweseli):. ± (+25 us ukukala inani lika-0.5%)
5. Ukufakwa: ukugeleza, ipayipi, ukucwiliswa
6. Ukufakwa kwamapayipi: imicu yamapayipi engu-1 ½ noma ¾ NPT
7. Umphumela: 4-20mA noma i-RS485
Ukuqhuba ugesi kuyindlela yokulinganisa ikhono lamanzi lokudlulisa ukugeleza kukagesi. Leli khono lihlobene ngqo nokugcwala kwama-ion emanzini 1. Lawa ma-ion aqhuba ugesi avela kusawoti oncibilikisiwe nezinto ezingaphili njenge-alkalis, ama-chloride, ama-sulfide kanye nama-carbonate compounds 3. Ama-compound ancibilikisa abe ama-ion aziwa nangokuthi ama-electrolyte 40. Uma ama-ion amaningi ekhona, kulapho ukuqhubela ugesi kwamanzi kukhuphuka khona. Ngokufanayo, ama-ion ambalwa asemanzini, kulapho ukudonsa amanzi kuncipha khona. Amanzi ahluziwe noma ahlanjululwe angasebenza njengesivikelo ngenxa yenani lawo eliphansi kakhulu (uma lingekho kangako) lokuqhuba ugesi 2. Ngakolunye uhlangothi, amanzi olwandle anokuqhuba okuphezulu kakhulu.
Ama-ion aqhuba ugesi ngenxa yokushaja kwawo okuhle nokubi 1. Lapho ama-electrolyte encibilika emanzini, ahlukana abe yizinhlayiya ezishajwe kahle (cation) kanye nezishajwe kabi (anion). Njengoba izinto ezincibilikisiwe zihlukana emanzini, amazinga eshaje ngayinye enhle nembi ahlala elingana. Lokhu kusho ukuthi yize ukuhanjiswa kwamanzi kukhuphuka ngama-ion engeziwe, ahlala engathathi hlangothi ngogesi 2
Umhlahlandlela Wethiyori Yokuqhuba Umoya
Ukulawula Ukushisa/Ukumelana nokushisa kuyipharamitha yokuhlaziya esetshenziswa kabanzi ekuhlaziyeni ukuhlanzeka kwamanzi, ukuqapha i-reverse osmosis, izinqubo zokuhlanza, ukulawula izinqubo zamakhemikhali, kanye nasemanzini angcolile ezimbonini. Imiphumela ethembekile yalezi zinhlelo zokusebenza ezahlukahlukene incike ekukhetheni inzwa yokulawula ukufudumala efanele. Umhlahlandlela wethu wamahhala uyithuluzi eliphelele lokubhekisela nokuqeqesha elisekelwe emashumini eminyaka obuholi embonini kulokhu kulinganisa.