Uchungechunge lwezimboni lwama-electrode okuqhuba ugesi lusetshenziswa ngokukhethekile ekulinganisweni kwenani lokuqhuba ugesi lamanzi ahlanzekile, amanzi ahlanzekile kakhulu, ukwelashwa kwamanzi, njll. Lufanelekela kakhulu ukulinganiswa kokuqhuba ugesi esitshalweni samandla okushisa kanye nasembonini yokwelapha amanzi. Lubonakala ngesakhiwo sesilinda esiphindwe kabili kanye nezinto ze-titanium alloy, ezingafakwa i-oxidized ngokwemvelo ukuze kwakhiwe i-chemical passivation. Ubuso balo bokuqhuba ugesi obungangeni bumelana nazo zonke izinhlobo zoketshezi ngaphandle kwe-fluoride acid. Izingxenye zesinxephezelo sokushisa yilezi: NTC2.252K, 2K, 10K, 20K, 30K, ptl00, ptl000, njll. ezichazwe ngumsebenzisi. I-electrode ye-K=10.0 noma i-K=30 isebenzisa indawo enkulu yesakhiwo seplatinum, emelana ne-asidi enamandla kanye ne-alkaline futhi inamandla amakhulu okulwa nokungcola; isetshenziswa kakhulu ekulinganisweni kwenani lokuqhuba ugesi ku-inthanethi ezimbonini ezikhethekile, njengemboni yokwelapha indle kanye nemboni yokuhlanza amanzi olwandle.
| I-electrode ehlala njalo | 0.1 | 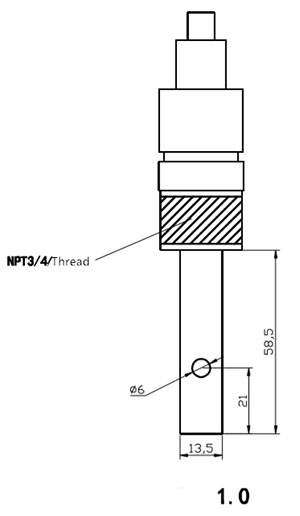 |
| Amandla okucindezela | 0.6MPa | |
| Ububanzi bokulinganisa | 0-200uS/cm | |
| Uxhumano | Ukufakwa Kwentambo Eyi-1/2 noma Eyi-3/4 | |
| Izinto | Insimbi engagqwali engu-316L | |
| Isicelo | Imboni Yokwelapha Amanzi |
Ukuqhubakuyisilinganiso sekhono lamanzi lokudlulisa ukugeleza kukagesi. Leli khono lihlobene ngqo nokuhlushwa kwama-ion emanzini
1. Lawa ma-ion aqhubayo avela kusawoti oncibilikisiwe nezinto ezingaphili njenge-alkalis, ama-chloride, ama-sulfide kanye nama-carbonate compounds
2. Ama-compound ancibilika abe ama-ion aziwa nangokuthi ama-electrolyte 40. Uma ama-ion amaningi ekhona, kulapho ukuhanjiswa kwamanzi kuphakama khona. Ngokufanayo, uma ama-ion ambalwa asemanzini, kulapho ukuhanjiswa kwamanzi kuncipha khona. Amanzi ahluziwe noma ahlanjululwe angasebenza njengesivikelo ngenxa yenani lawo eliphansi kakhulu (uma lingekho kakhulu). Amanzi olwandle, ngakolunye uhlangothi, anokuhanjiswa kwamanzi okuphezulu kakhulu.
Ama-ion aphehla ugesi ngenxa yokushaja kwawo okuhle nokubi
Uma ama-electrolyte encibilika emanzini, ahlukana abe yizinhlayiya ezishajwe kahle (cation) kanye nezishajwe kabi (anion). Njengoba izinto ezincibilikisiwe zihlukana emanzini, amazinga eshaje ngayinye enhle nembi ahlala elingana. Lokhu kusho ukuthi yize ukuhanjiswa kwamanzi kukhuphuka ngama-ion engeziwe, ahlala engathathi hlangothi ngogesi 2






















